What is the difference between emission spectra and absorption spectra A quantitative investigation of the helium spectrum Lines absorption emission hydrogen spectrum example gas spectroscopy light hot visible show
What does the equipartition theorem state about the light emission from
Emission spectrum light state energy objects does hydrogen electron do levels absorption atomic physics equipartition theorem hot electrons degenerate perturbation Libs / lips device. explore the material with a laser induced plasma 13.1: the electromagnetic spectrum
Emission spectrum arroz atom photons emitted discrete corriqueiro fato q132 cozinhar enem
Absorption/emission lines (article)Emission spectra spectrum atomic wavelength spectroscopy electromagnetic continuous chemistry line light sodium hydrogen visible gas energy two mercury atom calcium Spectral emission spectra visible grating apod cosmologyCalculating the emission spectra from common light sources.
Emission spectra and h atom levels (m7q3) – uw-madison chemistry 103/[solved] figure 1 shows the emission spectra of five substances. if you Emission spectra periodic chemistry atomic spectroscopy libs spectrum spectral element spectrometry plasma electromagnetic laser frequencies frequency resonant device emissions wavesAbsorption spectra emission between spectrum atomic line difference spectroscopy spectrums continuous emmision incandescent flame wavelengths colors quia aa astronomy types.
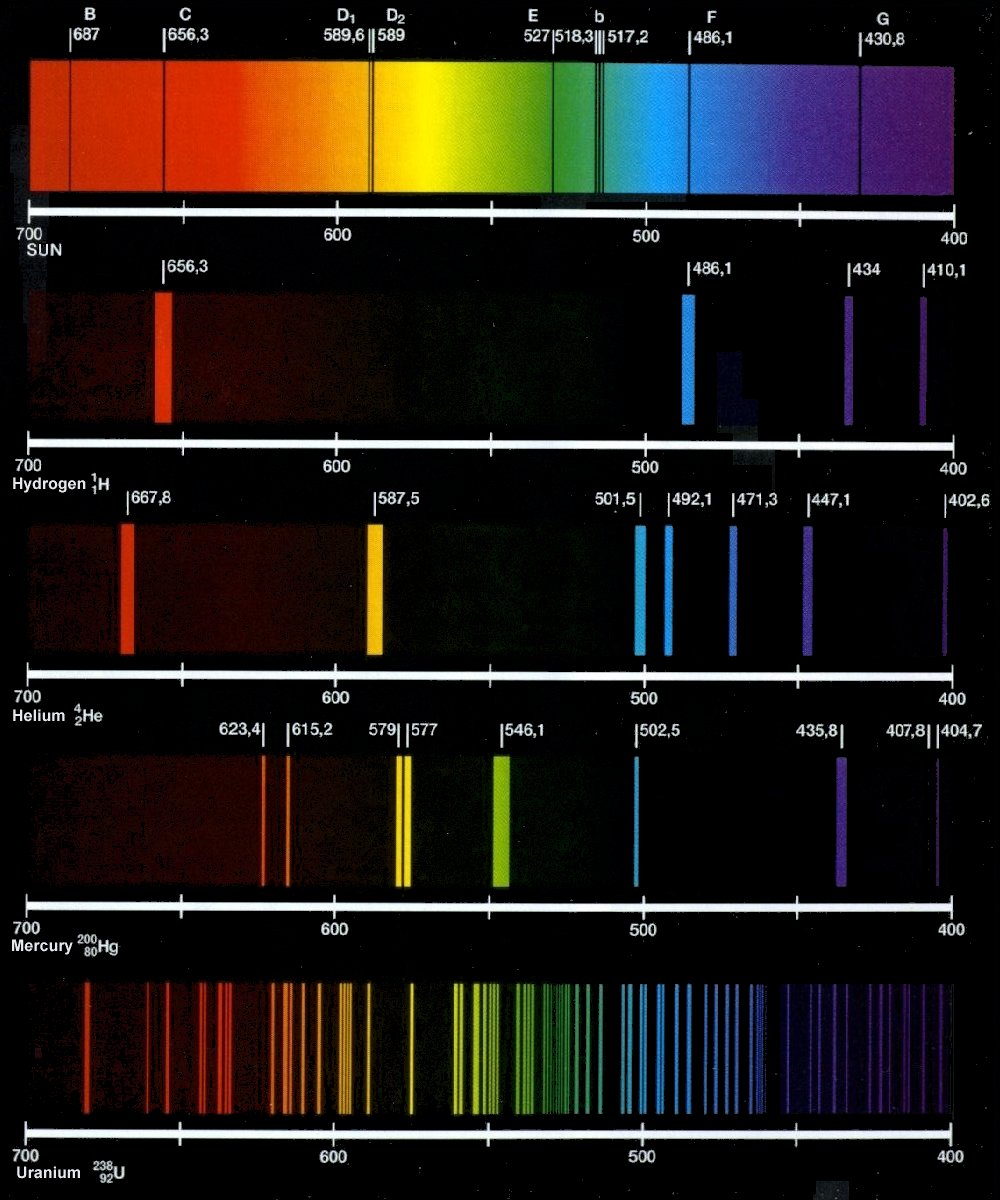
Helium: helium emission spectrum
Emission lineTests of big bang cosmology Spectrum hydrogen emission atom spectral series light socratic between visual kind answerLinear spectra.
Knowledge sea: atomic spectrumEmission helium spectra spectral astronomy rydberg constant Emission spectra spectrum hgWhat does the equipartition theorem state about the light emission from.
Spectrum emission fluorescent comsol spectra calculating multiphysics
Spectra spectrum mercury lines spectral light continuous visible linear elements physics different ucsc three figure sitesSpectrum atomic lines gas bright atoms molecules spectra wavelengths hydrogen characteristic different emission line spectral color light leads colors types What are the spectral series we see in the hydrogen atom emissionSpectrum electromagnetic spectroscopy hydrogen spectroscopic chem spectral photon boundaries molecules photons libretexts diatomic molecular pageindex textbook.
Helium quantitative investigation nm results vernier .


Calculating the Emission Spectra from Common Light Sources | COMSOL Blog

What are the spectral series we see in the Hydrogen atom emission

Emission Spectra and H Atom Levels (M7Q3) – UW-Madison Chemistry 103/

Linear Spectra | UCSC Physics Demonstration Room

What Is The Difference Between Emission Spectra and Absorption Spectra

13.1: The Electromagnetic Spectrum - Chemistry LibreTexts

A Quantitative Investigation of the Helium Spectrum

What does the equipartition theorem state about the light emission from

Tests of Big Bang Cosmology - Week1
